Introduction
SMD resistors are very popular in electronics. Their construction involves various materials, including silver (Ag), which is favored for its superior conductivity and resistance to oxidation. In SMD resistors, silver acts as a critical connection interface between the internal resistive layer and the external tin-plated terminal. However, despite its advantageous properties, silver contacts are susceptible to "sulfur attack" from the environment, potentially leading to significant reliability issues in electronic circuits. This vulnerability underscores the importance of meticulous design and protective measures in sulfur-prone environments.
The hidden vulnerability
In typical SMD resistors, there exists a seemingly minor but critical gap between the component coating layer and the tin-based terminal. Sometimes, there is no gap, but the coating is somehow porous, thus exposing the silver layer. This gap or pores, which might seem insignificant or easily overlooked, expose the silver layer to the external environment. In the photo below, you can see the SMD resistor, where I have marked the critical area.

This gap or porous area can expose the inner silver layer to the external environment. The following illustration shows a resistor cross-section. The red arrows indicate the location where this adverse phenomenon can occur:
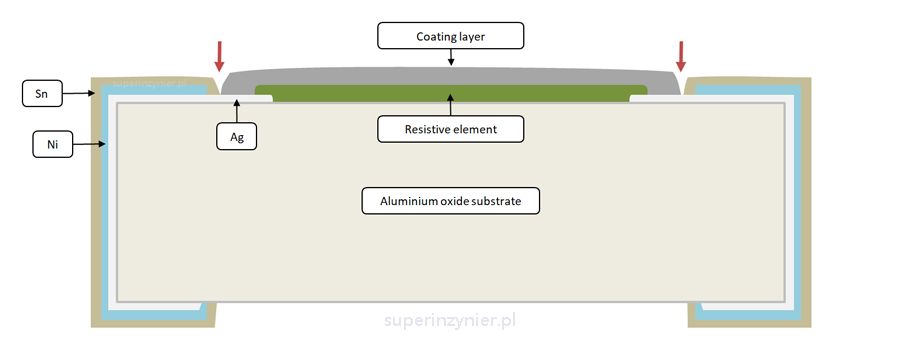
Sulfur containing gases
There are many types of sulfur-containing gases in the atmosphere. The most commonly known are hydrogen sulfide (H2S) or sulfur dioxide (SO2), which typically originate from automotive emissions, industrial processes, and the decomposition of organic matter. Typical sulfur-containing gases are:
- Hydrogen Sulfide (H2S). Industrial processes (e.g., rubber vulcanization[1], petroleum refining, natural gas processing), volcanic emissions, farms, and decomposition of organic matter.
- Sulfur Dioxide (SO2). Combustion of fossil fuels, volcanic eruptions, and industrial processes such as smelting.
- Carbonyl Sulfide (COS). Combustion of fossil fuels, volcanic emissions, production of synthetic fibers, and breakdown of organic sulfur compounds in soil.
- Dimethyl Sulfide (DMS, (CH3)2S). Marine phytoplankton emissions, bacterial decomposition of organic matter, industrial processes.
- Sulfur Hexafluoride (SF6). Electrical industry as an insulating gas, magnesium processing, and semiconductor manufacturing.
- Carbon Disulfide (CS2). Industrial processes like rubber vulcanization[2], volcanic emissions, and natural plant emissions.
- Methyl Mercaptan (CH3SH). Decomposition of organic matter, industrial processes (e.g., production of pesticides, plastics), and paper manufacturing.
The sulfur attack
When these sulfur-containing gases come into contact with the exposed silver layer, they react to form silver sulfide (Ag2S), a non-conductive compound. Example chemical reaction with hydrogen sulfide:
2Ag + H2S → Ag2S + H2
The picture below shows the ingress of sulfur (S) into the area where the silver layer (Ag) has contact with the air:

This chemical reaction gradually converts the exposed silver into silver sulfide. Once the exposed silver layer is transformed into Ag2S, the electrical connection is compromised, leading to resistor failure. Silver sulfide is a black-colored material, as shown below:

The photo below shows the "black spikes or feathers." It is the silver sulfide formed on the SMD resistor. The image from my recent electronics failure analysis:

Solutions
There are several strategies helpful to combat the sulfur attack problem.
Remove sulfur sources
The most common source of sulfur in a product design is a result of emission from rubber material[1] or vulcanized EPDM sponges. Review the product design, identify potential sources, and eliminate the source if possible. Check the following:
- Connectors with sealings
- Oils, lubricants
- Gaskets
- Rubber sealings
- Cooling fan filters
- Packing materials
- Vibration-isolating materials
Protect against sulfur
Applying organic coatings such as epoxy-based conformal coatings can create a physical barrier that prevents sulfur-containing gases from reaching the silver material. Do not use silicone-based coatings.
Use anti-sulfur parts
Standard SMD resistors may be replaced with "sulfur-resistant", "anti-sulfurated" or "anti-sulfur" parts, which will significantly increase product reliability. It is essential when the source of sulfur gas cannot be removed.
Summary
Sulfur poses a significant threat to the reliability of silver contacts in SMD resistors, leading to the formation of non-conductive silver sulfide and subsequent component failures. By understanding the failure mechanisms and identifying the sources of sulfur, engineers can take a proactive approach to mitigate this issue. Removing the sulfur sources, implementing protective coatings, and selecting sulfur-resistant components are effective strategies to enhance the durability and performance of electronic assemblies. This proactive approach ensures the longevity and reliability of products in various environmental conditions, putting you in control of the situation.
Footnotes
- R. Minamitani, "Estimation of Emission Characteristics of Sulfur Gas from Rubber," Journal of The Society of Materials Science, Japan, vol. 57, pp. 1114-1120, 2008. doi: 10.2472/JSMS.57.1114.
- L. Luo, R. Yuan, F. Liu, H. Yao, X. Yan, and H. He, "Emission evaluation of carbon disulfide from rubber surfaces in small environmental chambers," IOP Conference Series: Earth and Environmental Science, vol. 675, 2021, doi: 10.1088/1755-1315/675/1/012003.



